Abstract
Introduction Crovalimab, a novel C5 inhibitor, showed rapid and sustained terminal complement activity inhibition in patients with PNH who were C5 inhibitor-naive or switched from eculizumab in the Phase 1/2 COMPOSER study (Röth et al 2020). COMMODORE 3 (NCT04654468) is a Phase 3 multicenter single-arm trial studying crovalimab in C5 inhibitor-naive patients with PNH in China. Here, we report key COMMODORE 3 data.
Methods Chinese patients with PNH who were ≥ 12 years, ≥ 40 kg, had lactate dehydrogenase (LDH) ≥ 2 × upper limit of normal (ULN) and ≥ 4 packed red blood cell transfusions 1 year before screening were enrolled. Patients who previously received C5 inhibitor treatment were ineligible. Patients received crovalimab according to a weight-based dosing schedule, including loading (intravenous dose on Days 1 and 4, weekly subcutaneous doses starting from Day 2) and subcutaneous maintenance doses (every 4 weeks starting from Week 5); treatment continued after 24 weeks in patients with clinical benefit. Coprimary endpoints were: mean proportion of patients with hemolysis control (LDH ≤ 1.5 × ULN) from Week 5 through Week 25 and difference in proportion of patients who had transfusion avoidance from baseline through Week 25 vs within 24 weeks prior to screening. Secondary efficacy endpoints included breakthrough hemolysis, hemoglobin stabilization, and change in fatigue (Functional Assessment of Chronic Illness Therapy [FACIT]-fatigue scale). Safety and immunogenicity were also examined.
Results Patients enrolled (N = 51) had a median age of 31 years (range, 15-58; 3 patients 12-18 years) at baseline. Median time from PNH diagnosis was 7.1 years (range, 0.7-18.2). Nineteen patients (37%) had a history of aplastic anemia, and 1 (2%) had myelodysplastic syndrome. Mean PNH granulocyte clone size was 88.9% (standard deviation [SD], 13.7). Patients had a median of 16 packed red blood cell (pRBC) units (range, 8-51) transfused ≤ 1 year prior to screening. Mean LDH (× ULN) at baseline was 9.3 (SD, 2.8).
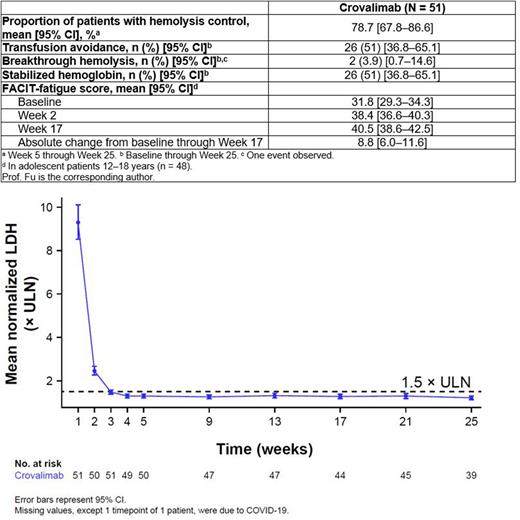
At clinical cutoff (February 10, 2022), both coprimary endpoints were met (Table). The mean proportion of patients achieving hemolysis control was 78.7% (95% CI, 67.8-86.6). Mean LDH reached ≤ 1.5 × ULN by Week 3 and was sustained through Week 25 (Figure). The difference between proportion of patients with transfusion avoidance from baseline through Week 25 (51.0%) vs within 24 weeks prior to screening (0%) was statistically significant at the two-sided 5% type I error level (P < 0.0001). The mean number of pRBC units transfused per patient decreased from 10.8 (SD, 6.6) during the 24 weeks prior to screening to 4.6 (SD, 6.7) from baseline through Week 25, with an average reduction in units transfused per patient of 6.1 (95% CI: 4.3-8.0). Breakthrough hemolysis was seen in 1 patient; another patient discontinued the study early and was conservatively considered to have breakthrough hemolysis. Twenty-six patients (51.0%) achieved hemoglobin stabilization. Clinically meaningful improvement in mean FACIT-fatigue score (≥ 5 points) was achieved by Week 2 and sustained through Week 17.
No adverse events led to treatment discontinuation. One patient had a Grade 5 adverse event (subdural hematoma after fall; not treatment-related). Grade 3-4 clinical adverse events were bile duct stone, abdominal wall mass, and bacteremia (1 patient each). Clinical adverse events occurring in ≥ 10% of patients were upper respiratory tract infection (24 patients [47.1%]; all Grade 2, not serious and resolved without dose change) and weight increased (6 patients [11.8%]). No patient had meningococcal infection. Treatment-emergent anti-drug antibodies were detected in 16 patients (31.4%). No patient developed neutralizing antibodies. There is no evidence for different efficacy and safety in adolescent patients 12-18 years.
Conclusions COMMODORE 3 met both coprimary endpoints of hemolysis control and transfusion avoidance, with no new safety signals, demonstrating that crovalimab is efficacious and well tolerated in patients with PNH.
Disclosures
Xia:F. Hoffmann-La Roche Ltd.: Other: All authors received support for third-party writing assistance, furnished by Bena Lim, PhD, of MediTech Media and funded by F. Hoffmann-La Roche Ltd, Basel, Switzerland., Research Funding. Weng:F. Hoffmann-La Roche Ltd.: Other: All authors received support for third-party writing assistance, furnished by Bena Lim, PhD, of MediTech Media and funded by F. Hoffmann-La Roche Ltd, Basel, Switzerland., Research Funding. He:F. Hoffmann-La Roche Ltd.: Other: Medical writing support, furnished by Bena Lim, PhD, of MediTech Media, and funded by F. Hoffmann-La Roche Ltd, Basel, Switzerland, Research Funding. Chang:Genentech, Inc.: Current Employment; F. Hoffmann-La Roche Ltd: Current equity holder in publicly-traded company, Other: Medical writing support, furnished by Bena Lim, PhD, of MediTech Media, and funded by F. Hoffmann-La Roche Ltd, Basel, Switzerland. Lundberg:F. Hoffmann-La Roche Ltd.: Current Employment, Other: Medical writing support, furnished by Bena Lim, PhD, of MediTech Media, and funded by F. Hoffmann-La Roche Ltd, Basel, Switzerland. Sima:F. Hoffmann-La Roche Ltd.: Other: Medical writing support, furnished by Bena Lim, PhD, of MediTech Media, and funded by F. Hoffmann-La Roche Ltd, Basel, Switzerland; Genentech, Inc.: Current Employment. Shi:Roche Product Development: Current Employment; F. Hoffmann-La Roche Ltd: Other: Medical writing support, furnished by Bena Lim, PhD, of MediTech Media, and funded by F. Hoffmann-La Roche Ltd, Basel, Switzerland. Xiao:Roche Product Development: Current Employment; F. Hoffmann-La Roche Ltd: Other: All authors received support for third-party writing assistance, furnished by Bena Lim, PhD, of MediTech Media and funded by F. Hoffmann-La Roche Ltd, Basel, Switzerland.. Zhang:F. Hoffmann-La Roche Ltd.: Other: All authors received support for third-party writing assistance, furnished by Bena Lim, PhD, of MediTech Media and Scott Battle, PhD, of Health Interactions and funded by F. Hoffmann-La Roche Ltd, Basel, Switzerland; China Innovation Center of Roche: Current Employment. Zhang:F. Hoffmann-La Roche Ltd.: Other: Medical writing support, furnished by Bena Lim, PhD, of MediTech Media, and funded by F. Hoffmann-La Roche Ltd, Basel, Switzerland; Roche Product Development: Current Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal